Background:
TGRX-678 is a novel allosteric inhibitor of ABL kinases, specifically targeting the ABL Myristoyl Pocket (STAMP). In vitro data supports TGRX-678 targeting the most common BCR-ABL mutations, including T315I. Here we report an open-label, first-in-human study to evaluate the safety, preliminary efficacy, and pharmacokinetic properties of orally administered TGRX-678 in patients with resistant/refractory CML.
Methods:
This study included dose escalation and expansion phases. During dose escalation patients with CML in chronic or accelerated phase (CP or AP) and who had failed ≥3 prior TKIs were enrolled. Patients received orally a single dose, after a 3-5 day observation, continuously daily doses ranging from 10 to 80 mg (BID) or 40 to 240 mg (QD) were given. Dose escalation followed an adaptive Bayesian logistic regression model based on dose-limiting toxicities (DLTs) occurring in cycle 1 (28 Days). Dose-expansion had three cohorts: CML-CP without T315I mutation with ≥2 prior TKIs, CML-CP with T315I with ≥1 prior TKIs, and CML-AP with ≥1 prior TKIs. The eligible patients received continuous treatments until disease progression, intolerant toxicity, consent withdrawal, or death.
Results:
From April 30, 2021 to July11, 2023, 95 (CP n = 58, AP n = 37) patients were treated with TGRX-678 at the BID doses including 10 mg (n = 3), 20 mg (n = 6), 40 mg (n = 5), and 80 mg (n = 3), and QD doses including 40mg (n=18), 80 mg (n = 6), 160mg (n= 4) and 240mg (n=50). 40 (42%) patients were female. The median age was 45 (range 35-52) years and the median interval from diagnosis to initial TGRX-678 treatment was 97 (range 28-136) months. Median treatment duration was 8 (range 6-13) months. At baseline, 93 (98%) patients had received ≥2 lines of prior TKI therapy, among them, 78 (82%) received ≥3 lines of prior TKIs, 44 (46%) received ≥4 lines of prior TKIs. 46 (48%) patients had ≥1 ABL mutation and 28 (30%) had T315I mutation.
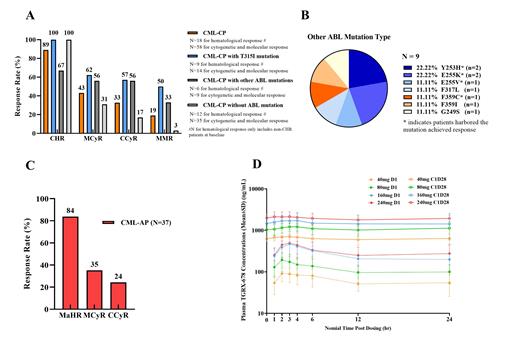
Of 58 patients with CML-CP, 16 (89%) achieved complete hematologic responses (CHR), 25 (43%) major cytogenetic responses (MCyR), 19 (33%) complete cytogenetic responses (CCyR), and 11 (19%) major molecular responses (MMR). Of 14 CML-CP patients with T315I mutation, 9 (100%) achieved CHR, 9 (62%) MCyR, 8 (57%) CCyR and 7 (50%) MMR. Of 35 CML-CP patients without any ABL mutations, 12 (100%) achieved CHR, 11 (31%) MCyR, 6 (17%) CCyR and 1 (3%) MMR (Fig. A). Of 9 CML-CP patients with other ABL mutations, 4 (67%) achieved CHR, 5 (56%) MCyR, 5 (56%) CCyR and 3 (33%) MMR (Fig. B). Of 37 CML-AP patients, 31 (84%) had major hematologic response, 13 (35%) MCyR, and 9 (24%) CCyR (Fig. C).
There were 22 CML-CP and 23 CML-AP patients previous received 3 rd Gen TKIs (ponatinib or HQP1351) or asciminib. Of 22 CP patients, 21 (95%) achieved CHR, 4 (18%) MCyR and 3 (14%) CCyR. Of 23 CML-AP patients, 17 (74%) achieved CHR, 5 (22%) MCyR and 3 (13%) CCyR.
The PK results suggested that TGRX-678 exposure (C max and AUC tau) was dose-proportional within range of 10mg~80mg BID or 40mg~240mg QD. The elimination of TGRX-678 in human plasma is slow, and the half-life (T1/2) of which is about 120 hours. At steady state, the accumulation ratios of C max and AUC tau are 3.7-8.0 and 5.4-11.8, respectively. The high accumulation ratio of TGRX-678 resulted in not only an increase of plasma exposure, but also a decrease of the gap between C max and C trough (Fig. D).
7 DLTs were observed, one case of elevated alanine aminotransferase at 20 mg BID and five cases of thrombocytopenia at 40mg QD (1), 40mg BID (2) and 80mg QD (2) and one hepatic function abnormal at 240 mg QD. However, MTD was not reached according to protocol criteria. Most treatment-related adverse events (TRAEs) were grade 1-2. AEs ≥ grade 3 that happened more than 5% were thrombocytopenia (50%), neutropenia (42%), anemia (24%) and hypertriglyceridemia (9%). In total 15 patients discontinued the study due to disease progression (5, all APs), intolerance (2), physician's decision (2), consent withdrawal (6). There was one death occurred which was not drug related.
Conclusions:
Clinical activity of TGRX-678 was seen in all cohorts and across TKI-resistant mutations including T315I, providing a promising treatment option for CML CP/AP patients, including those who failed ponatinib or asciminib. Its unique PK properties might bring additional benefit to patients. TGRX-678 was well tolerated in heavily pretreated CML patients. Currently two doses were selected to optimize the RP2D.
Study Identifier: NCT05434312.
Disclosures
Wang:Shenzhen TargetRx, Inc.: Current Employment, Current equity holder in private company, Other: CEO of the company. Cao:Shenzhen TargetRx, Inc.: Current Employment, Current equity holder in private company, Other: COO of the company.